our technology
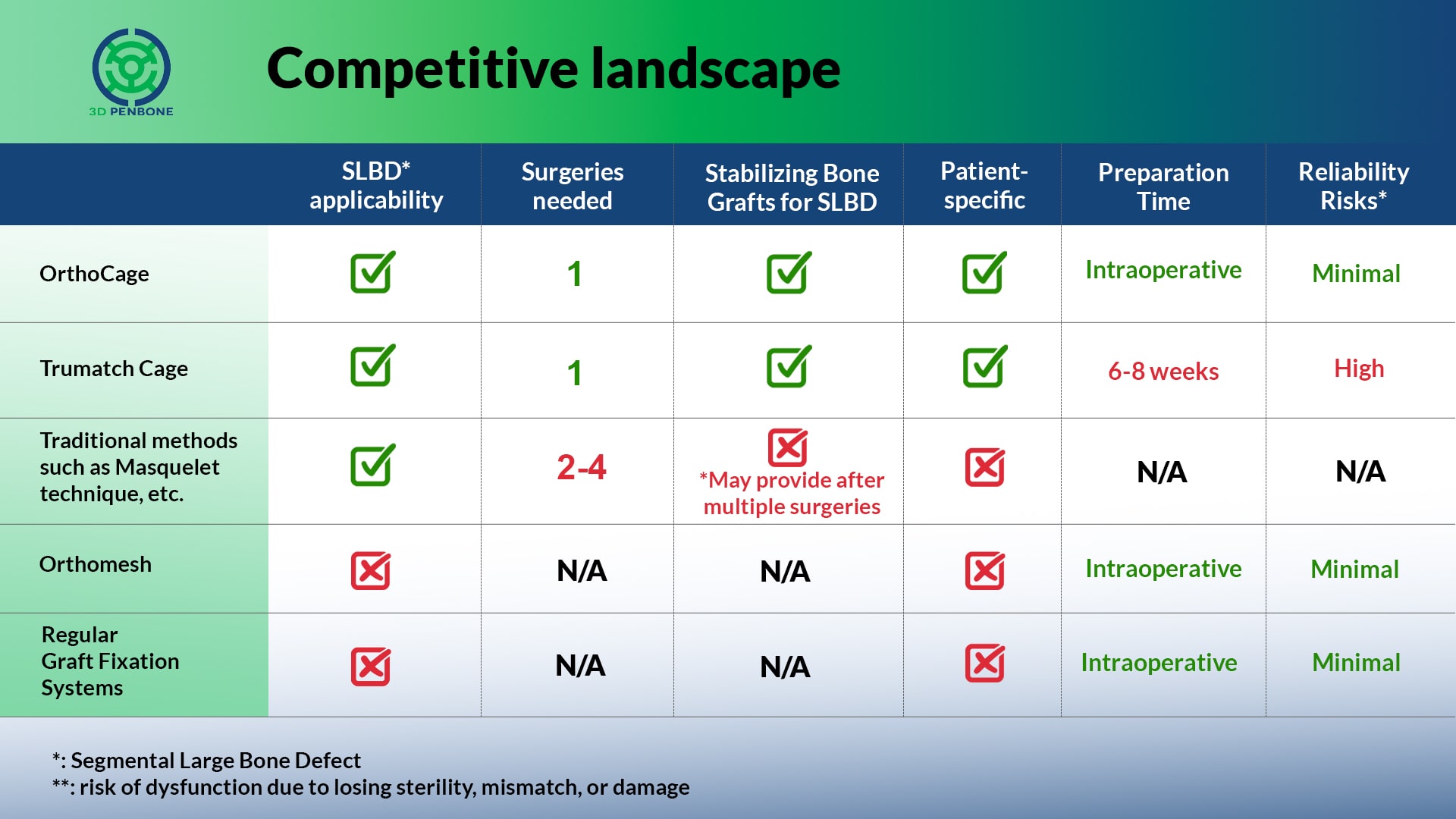
OrthoCage
Innovative solutions for treating orthopedic defects, especially segmental large bone defects.
INCREASE the chance of using bone grafts for segmental large bone defects
REDUCE the chance of amputation
REDUCE complexity and numbers of the surgeries
SHORTEN the recovery time
IMMEDIATELY being ready-to-use intraoperatively
ELIMINATES the risk of dysfunction due to damage, distort or mismatch
ENABLES patient-specific solution without any delay
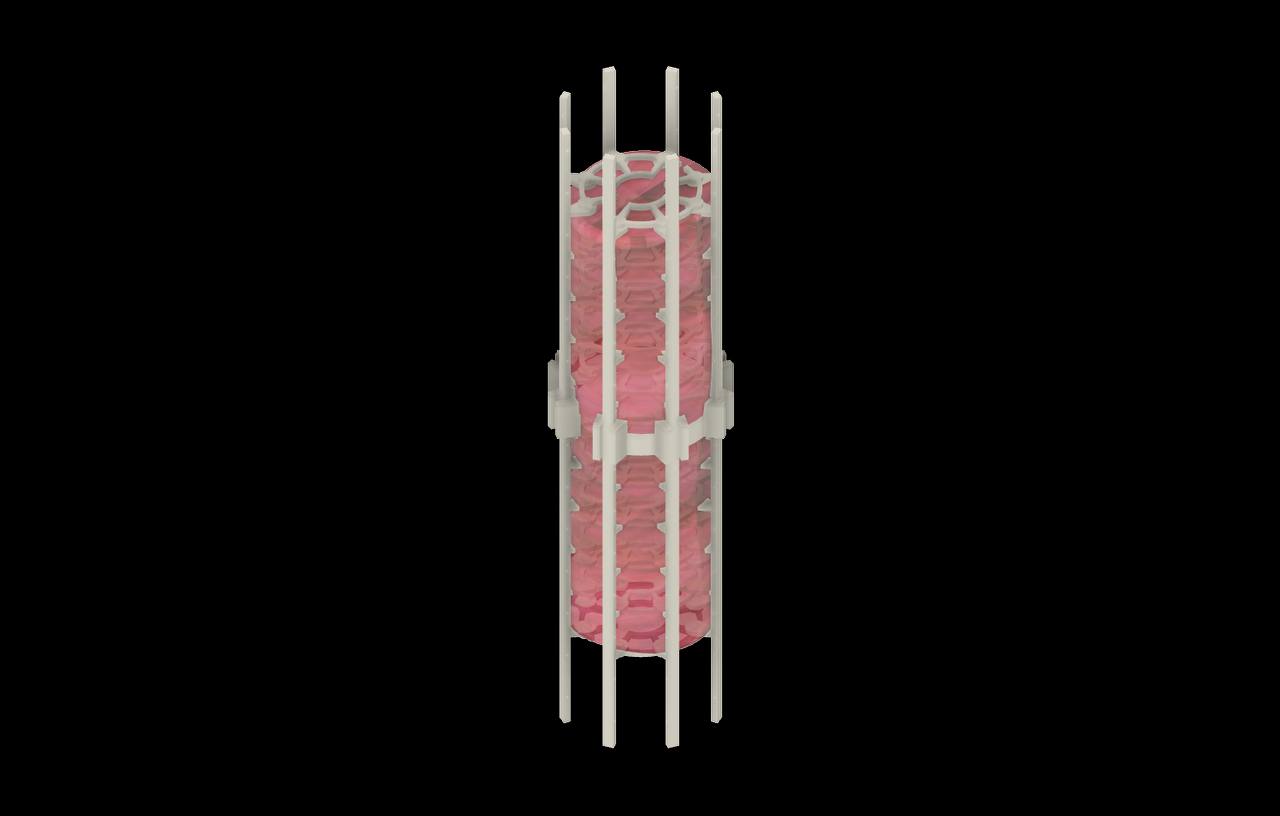
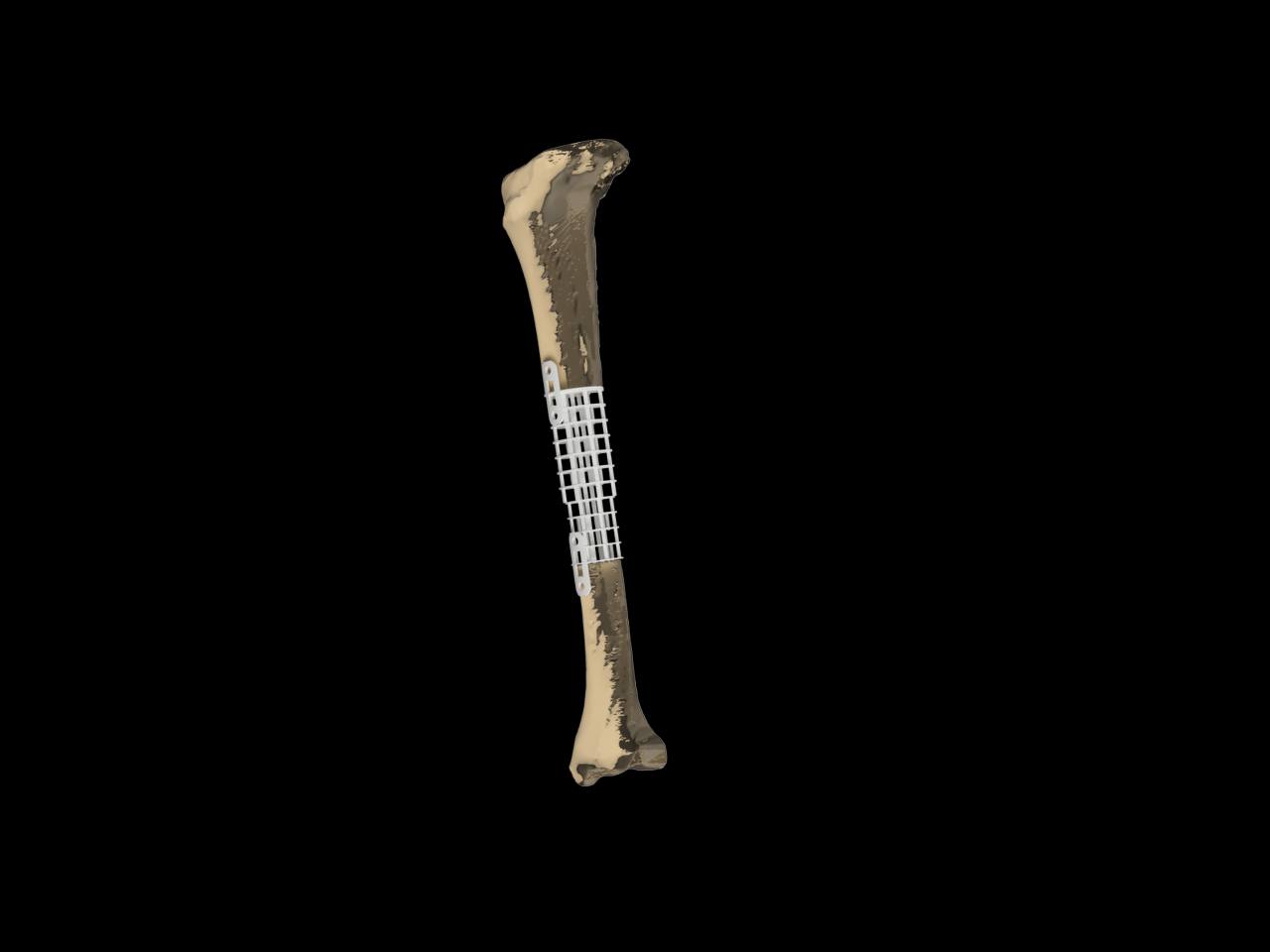
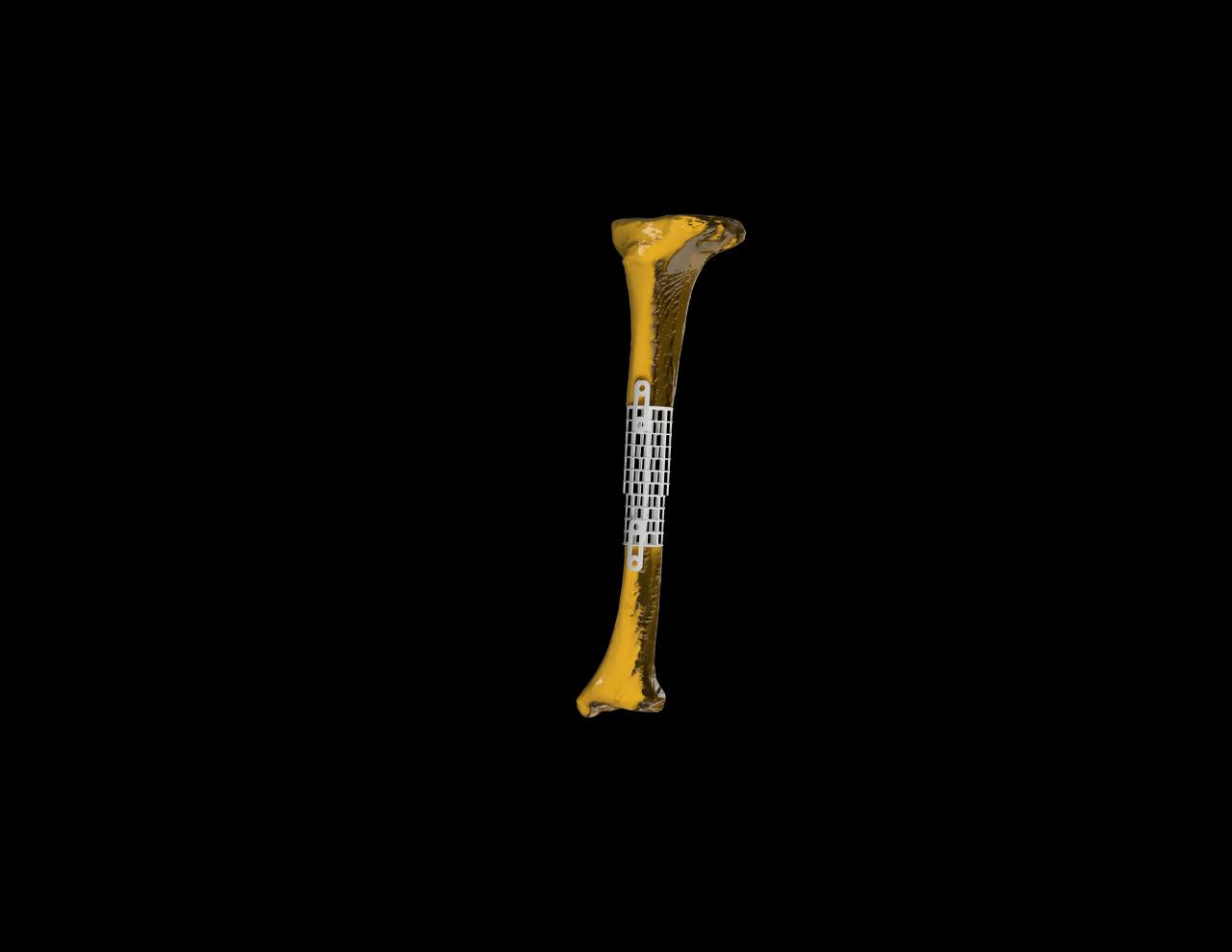
Features
OrthoCage has a confirmed straightforward 510(k) FDA regulator pathway
The timeline for the FDA pathway of OrthoCage:
1
Pre-submission for 510(k)
2
Meeting with FDA reviewers
3
FDA confirmed 510(k) pathway
4
510(k) Submission
5